

Why Blueneem?
Patient safety is our top priority. To ensure our medical devices are designed and built to the highest safety standards, we have integrated a robust risk management framework, over and above all regulatory requirements, into our quality assurance processes.
Our MedTech devices are designed to minimize potential patient harm in all situations. However, any medical process carries a degree of risk. To mitigate this, we also implement post-market surveillance systems to continuously monitor and assess the performance of our products and identify any potential safety issues. Our team also works closely with healthcare providers to educate them on the proper use of our devices and provide timely warnings and updates on any post-market safety concerns.
This risk management framework lies at the heart of our MedTech product development operations and greatly influences both product and process decisions.
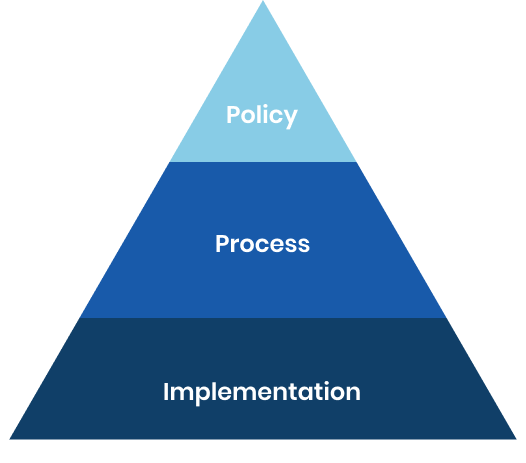

A robust risk management policy has been created in consultation with industry experts to ensure that objectives, patient well-being priorities, regulatory and statutory requirements, state-of-the-art methodology, and clinician expectations are all met.
Our risk management policy includes provisions for monitoring and reviewing the effectiveness of the controls and decisions made, as well as analyzing and quantifying risk while mitigating ambiguities and uncertainties.
Blueneem employs a robust risk management process to ensure that our products are safe. We are driven by a more powerful motivator- the joy of a recovered patient and the smile of a satisfied surgeon.
Every function and every member of the Blueneem family are centered around one philosophy – Patient Wellbeing. It is embedded in our DNA.
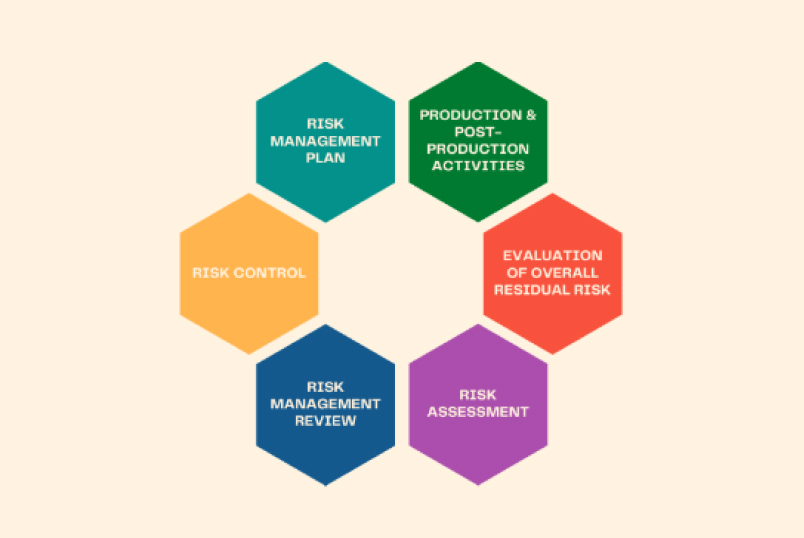

To achieve efficiency, our risk management process revolves around the following methods:
FMEA & FTA – Identification of hazards
Probability X Severity matrix
Feedback mechanism and data analysis
Understanding the precise intended use of the device
to know the precise device requirements to meet the intended use
to identify the risks related to design, production and information
Performing Clinical investigation or evaluation to validate that the device will cater/meet its intended use, risk free and user friendly[usability]
Design verification, Biocompatibility testing and Microbial testing
to mitigate the identified risks to acceptable levels as low as reasonably possible [ALARP]
Controlled design changes if required without compromising the performance of the device
Adherence to product specification by process validation, conformance of product specs by incoming, inprocess and final quality control
Product certification and licensing for assurance to user regarding safety of device [CE 0123]
Blueneem, a globally recognized leader in MedTech design and manufacturing, strives to create an ecosystem of compassionate disruption. With more than 40 products developed and delivered into the hands of clinicians in 70-plus countries, our focus is on incremental innovation, meeting the requirements of medical professionals, and improving patients’ lives.
Innovation Hub with End-to-End Design & Development
We listen to our doctors intently. As their innovation partner, we explore new avenues in designing, developing, and manufacturing minimally invasive medical devices to deliver optimum patient care and outcome.
What we do
We leave no stone unturned while developing innovative products. We search high and low to find the right solutions. We don’t shy away from burning the midnight oil when it comes to designing breakthrough tools. And we are always on our feet developing and delivering value to our doctors and clinician stakeholders.


Our end-to-end manufacturing process – from sourcing raw materials to delivering the finished product – ensures quality and efficiency throughout the production cycle.



