This article is about a randomized trial investigating clinical outcomes and stent-related symptoms after placing a CIUS on a string versus conventional stent placement.
For over 30 years, the JJ ureteric stent has been widely used in urological procedures since its invention in 1978. However, the conventional ureteric JJ stent (CUS) placement has been associated with discomfort and a reduction in patient quality of life.
Tailed stents have been proposed as an alternative due to fewer stent-related symptoms and lower costs, but the perfect ureteric stent design has not yet been achieved.
Clinical Study conducted by:
Department of Urology and Renal Transplant, Kasturba Medical College, MAHE, Manipal, Karnataka, India, and Istanbul Medipol Mega University Hospital, Istanbul, Turkey
Topic:
Investigating clinical outcomes and stent-related symptoms (SRS) after placement of a complete intra-ureteric stent (CIUS) on a string versus conventional stent placement.
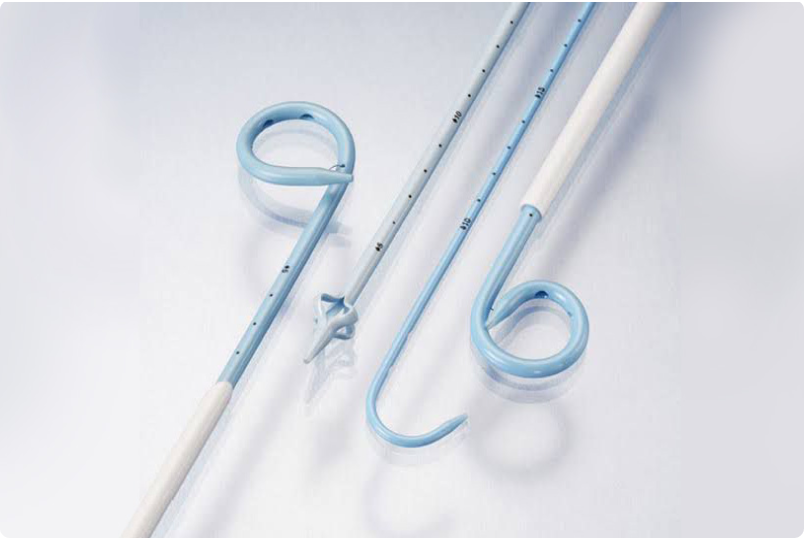
In this study, the authors have aimed to evaluate the safety and effectiveness of a novel approach called a complete intra-ureteric stent (CIUS) placement compared to CUS placement. The CIUS involves positioning the distal end of the ureteric stent above the ureteric orifice, while CUS places it within the bladder.
Blueneem’s products covered in the study:
Kidsoft® Hypospadias stent Single J
P Flex Ureteral Stent (D J Stent)
Conclusion:
The use of a CIUS (complete intra-ureteric stent) with extraction suture decreases SRS (stent-related symptoms) after URS (Ureteroscopy).










